Effect of metformin on clinical gout outcomes in gout patients with diabetes mellitus
F. Veenstra (1), Lm. Verhoef (1), M. Opdam (1), Aa. Den Broeder (1,3), W. Kwok (2), I. Meek (3, Fhj. Van Den Hoogen (1,3), M. Flendrie (1), N. Van Herwaarden (1,3)
Affiliation(s):
1. Department of Rheumatology, Sint Maartenskliniek, Nijmegen, The Netherlands;
2. Department of Rheumatology, Rijnstate Hospital, Arnhem, The Netherlands;
3. Department of Rheumatology, Radboud University Medical Center, Nijmegen, The Netherlands
Background: Gout and diabetes mellitus type 2 (DM) are frequently co-existing. Metformin is the first choice of treatment for patients with DM type 2, and might – based on previous studies - have beneficial clinical effects on gout through a putative anti-inflammatory as well as serum uric acid (SUA) lowering effect [1,2,3] . Objectives: To investigate the anti-inflammatory and SUA lowering effect of metformin in patients with gout starting uric acid lowering treatment (ULT).
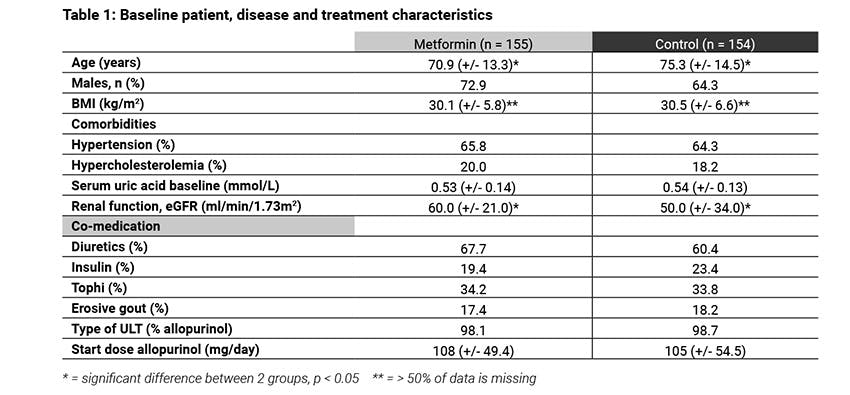
Methods: Patients with clinical diagnosis of gout, a first outpatient visit between January 2010 and March 2018 and a follow-up of at least 6 months were included in a retrospective cohort study, conducted in two rheumatology centres in the Netherlands. From this cohort patients with DM starting ULT were selected. Patients with metformin use were compared to patients using other or no antidiabetic medication (control group). Metformin use was defined as use ≥80% of the time during the first six months after initiation of ULT. To evaluate the anti-inflammatory effect, the differences in incidence density (ID) of gout flares in the first six months after starting ULT was measured and analysed using Poisson regression. To evaluate the SUA lowering effect, the difference in baseline SUA, proportion of patients reaching target serum uric acid (SUA < 0.36 mmol/l) at six months follow up, and ULT dosage at time of reaching this target were analysed. All analyses included correction for confounding.
Results: Of 2108 gout patients, 309 patients who started ULT also had DM, with 155 in the metformin group and 154 in the control group. ID of flares was 2.8 and 3.3 per patient year in the control and metformin group respectively, resulting in an incidence rate ratio of 0.93 (95% CI 0.75 – 1.15). SUA levels at baseline were similar, between the two groups (Table 1, p=0.31). At six months 46.1% and 57.4% reached target SUA in the control and metformin group respectively, odds ratio of 1.30 (95%CI 0.80 – 2.08). No difference was found in allopurinol dose at time of reaching target SUA between the groups (difference: 1.14 mg, 95% CI -38.89 – 41.19).
Conclusions: In contrast to a previous report [1], in this study in patients with DM and gout and starting ULT, metformin does not have a clinically relevant added anti-inflammatory or urate lowering effect.
References: 1. Vazirpanah N et al. Ann Rheum Dis. 2019;78(5):663-71. 2. Simao AN et al. Expert opinion on therapeutic targets. 2012;16(12):1175-87. 3. Barskova VG et al. Klin Med (Mosk). 2009;87(7):41-6.