Urate lowering effect of febuxostat in relation to renal function and starting dose in gout patients in daily clinical practice ; a single-centre experience
Marcel Flendrie, H.A. Martens, Cornelia Van Den Ende, J.T.H Van Zadelhoff, F.H.J. Van Den Hoogen
Affiliation(s):
Department of Rheumatology, Sint Maartenskliniek, Nijmegen, The Netherlands
Introduction : Febuxostat is considered to be safe in gout patients with moderate to severe renal damage, but data are limited. Doctors might start with a more cautious dose of 40 mg in these patients for safety reasons instead of the registered 80 mg.
Objective : To explore differences in urate-lowering effect and safety of febuxostat in patients with and without renal impairment.
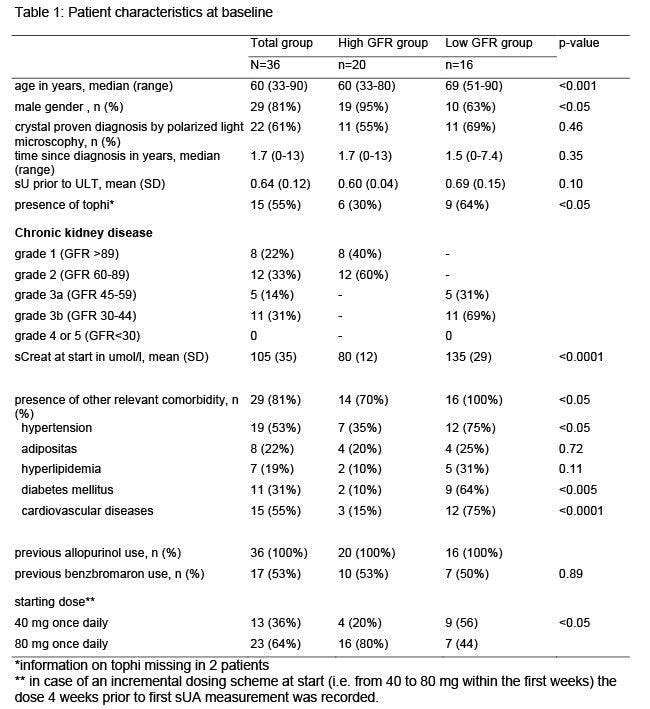
Methods : All gout patients starting febuxostat at a single tertiary care centre were included in this retrospective study. Patients and disease characteristics at baseline were extracted from patient charts, as well as starting date and dose of febuxostat and relevant comorbidity (hypertension, obesity, diabetes mellitus, hyperlipidemia, cardiovascular disease). Over time dose changes with dates and reasons were collected, as well as serum urate (sU) and creatinin (sCreat). Patients were divided in two groups according to the glomerular filtration rate (GFR), calculated with the MDRD formula: GFR below 60 ml/min/1.73m2 (Low GFR group) or not (High GFR group).
Baseline characteristics were compared by Chi-square or independent T-test, when appropriate. Mean sU and mean sCreat over time per group were calculated. Percentual change from baseline and percentage of patients reaching a target below 0.36 mmol/l within 12 weeks were calculated. Changes in sU within 12 weeks from baseline were compared by one-sided paired sample t-test in both groups.
Results : Thirty-six gout patients started on febuxostat between April 1st 2012 and July 1st 2017. Sixteen (44%) had a GFR below 60 ml/min/1.73m2. All had failed on allopurinol and 53% on benzbromaron. Baseline characteristics are presented in table. The Low GFR group counted significantly more often comorbidity (100% vs 61%, with more diabetes, hypertension and cardiovascular disease) and more often had tophaceous gout (64% vs 30%). Thirteen patients started on 40 mg/day (9 in Low GFR group) and 23 on 80 mg/day (7 in Low GFR group).
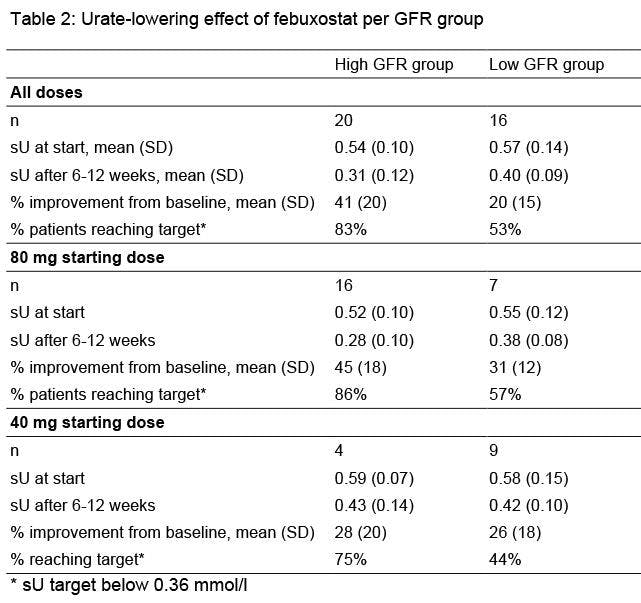
In the High GFR group mean sU dropped from 0.54 (SD =0.10) at baseline to 0.31 (0.12) after 6-12 weeks, with a mean improvement of 41% from baseline and 83% reaching target. In the Low GFR group mean sU dropped from 0.57 (SD=0.14) at baseline to 0.40 (0.09) after 6-12 weeks, with 28% mean improvement from baseline and 53% of patients reaching target. Table two shows urate lowering-effects of both febuxostat starting doses per GFR group. Median follow-up time for all patients was 1.6 years (range 0-6.7 years). For the High GRF group respectively Low GFR groups follow-up time was 1.4 y (0-6.7 y) resp. 1.7 (0.4-5.5 y). Five of 36 patients (14%) stopped during follow-up (3 in the Low GFR group), all within two months after start and all because of adverse events: diarrhea (n=2, 40 mg and 80 mg), coronary syndrome (n=1, 80 mg), erectile dysfunction (n=1, 40 mg) and gout attacks (n=1, 40 mg). Other possible related adverse events reported were dry mouth (n=1, 80 mg), elevated liver enzymes (n=1, 40 mg) and flushing ( n=1, 40 mg).
Conclusions : A starting dose of febuxostat of 80 mg/day dosing appears to be safe and to result in a stronger reduction of serum urate than 40 mg/day, in patients with normal or with mild to moderate renal impairment (GFR 30 to 60 ml/min/1.73m2).