Genetic loci that affect urate levels but not gout risk – an apparent discordance between urate and gout genetics.
Riku Takei, Nicholas Sumpter, Tony Merriman
Affiliation(s):
University of Alabama at Birmingham
Objective: To investigate the differences and similarities between the genetic loci identified in gout and urate genome-wide association studies (GWAS), and to identify loci that have disproportionate effects on gout relative to the effects on serum urate.
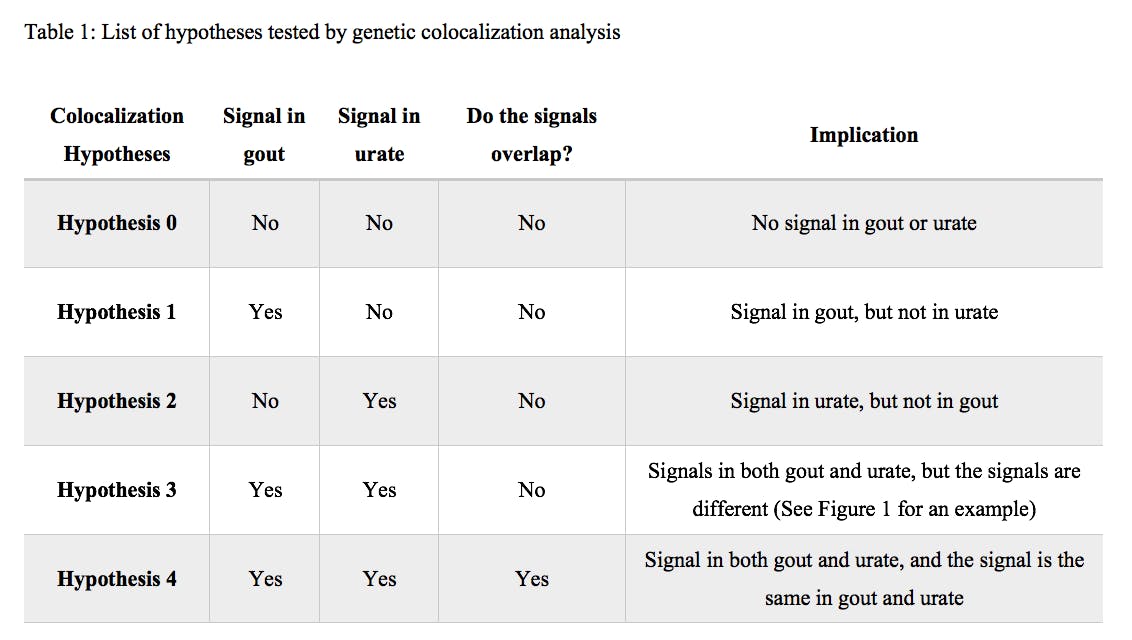
Methodology: GWAS of gout1 and urate1,2 were carried out in people of European ancestry and genetic loci were identified based on the lead signals of each of the GWAS. Linkage Disequilibrium Score Regression (LDSC)3,4 was used to calculate the genetic correlation between gout and urate. Genetic colocalization of gout and urate was first carried out using 291 gout-associated lead variants and then repeated with 422 serum urate-associated lead variants, using the ‘coloc’ package in R5. The region for colocalization was defined as the lead variant ±500kb (1Mb window around the lead variant). For any of the hypotheses of colocalization (Table 1), posterior probability (PP) ≥0.8 was used as the cutoff to determine whether the genetic locus was significant for that hypothesis.
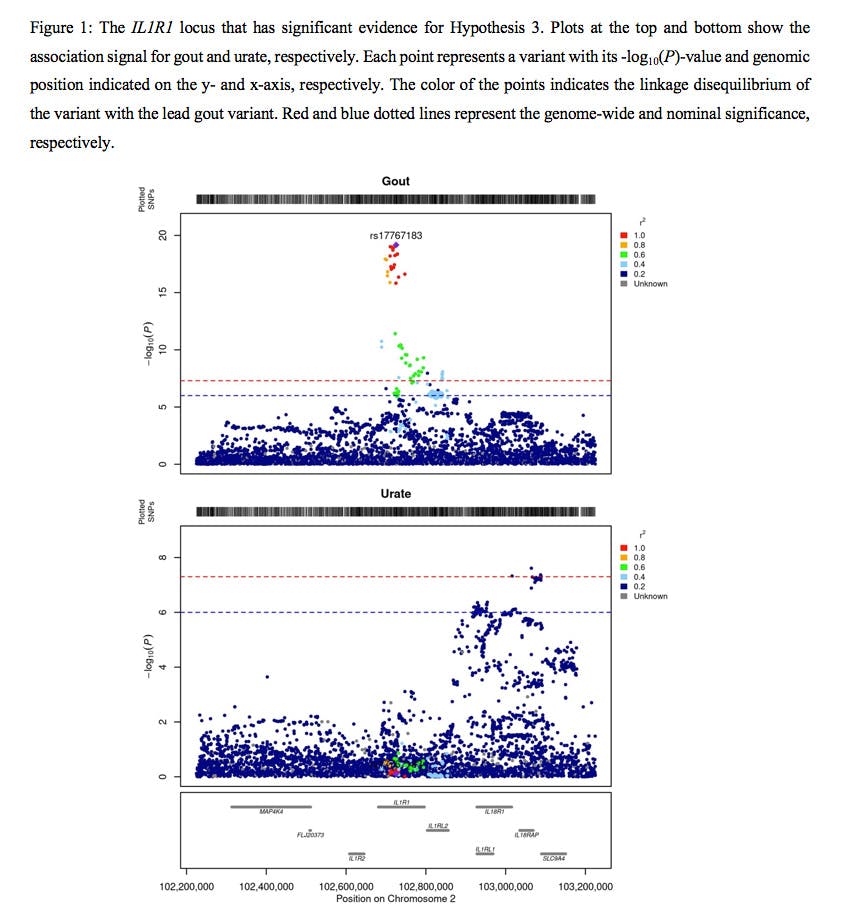
Results: Genetic correlation analysis of gout with serum urate levels showed high, but not perfect, correlation (rg = 0.89). Colocalization analysis of gout and urate lead variants revealed 162 and 183 loci, respectively, that had significant evidence of shared signal between gout and urate (PP for hypothesis 4 (H4) ≥0.8). For the gout lead variants, there were nine that were specific to gout (PPH1 ≥0.8 and Purate >0.01) and six that showed different association signals in gout and urate (PPH3 ≥0.8 and Purate >0.01; Figure 1). Using a similar approach, there were 18 loci that were associated only with urate (PPH2 ≥0.8 and Pgout >0.01) and 10 with different signals (PPH3 ≥0.8 and Pgout >0.01) for the urate lead variants.
Conclusions: Elevated serum urate level is an established risk factor of gout, and the formation of monosodium urate crystals is essential and causal of acute gout inflammation. Logically, gout genetic loci that are not associated with urate level are involved in gout-specific mechanisms (such as crystal formation and the inflammatory response), and genetic loci that affect serum urate levels should also affect the risk of gout. However, we show that there are genetic loci that have genome-wide significant association with urate, but have weak association with the risk of gout, suggesting the possibility of genetic loci that affect gout risk through two (or more) pathways that work in opposing direction. In this scenario, a specific urate-increasing allele will affect gout risk through increased urate, but also decreases gout risk through an alternate pathway in such a way that nullifies the risk of gout. The presence of such genetic loci will help identify novel biological mechanisms of gout.
References:
1. Major, T. J. et al. A genome-wide association analysis of 2,622,830 individuals reveals new pathogenic pathways in gout. Preprint at https://doi.org/10.1101/2022.11.26.22281768 (2022)
2. Tin, A. et al. Target genes, variants, tissues and transcriptional pathways influencing human serum urate levels. Nature Genetics 51, 1459–1474 (2019).
3. Bulik-Sullivan, B. K. et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nature Genetics 47, 291–295 (2015).
4. Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nature Genetics 47, 1236–1241 (2015).
5. Giambartolomei, C. et al. Bayesian Test for Colocalisation between Pairs of Genetic Association Studies Using Summary Statistics. PLOS Genetics 10, e1004383 (2014).